Descriptive Studies
What are descriptive studies?
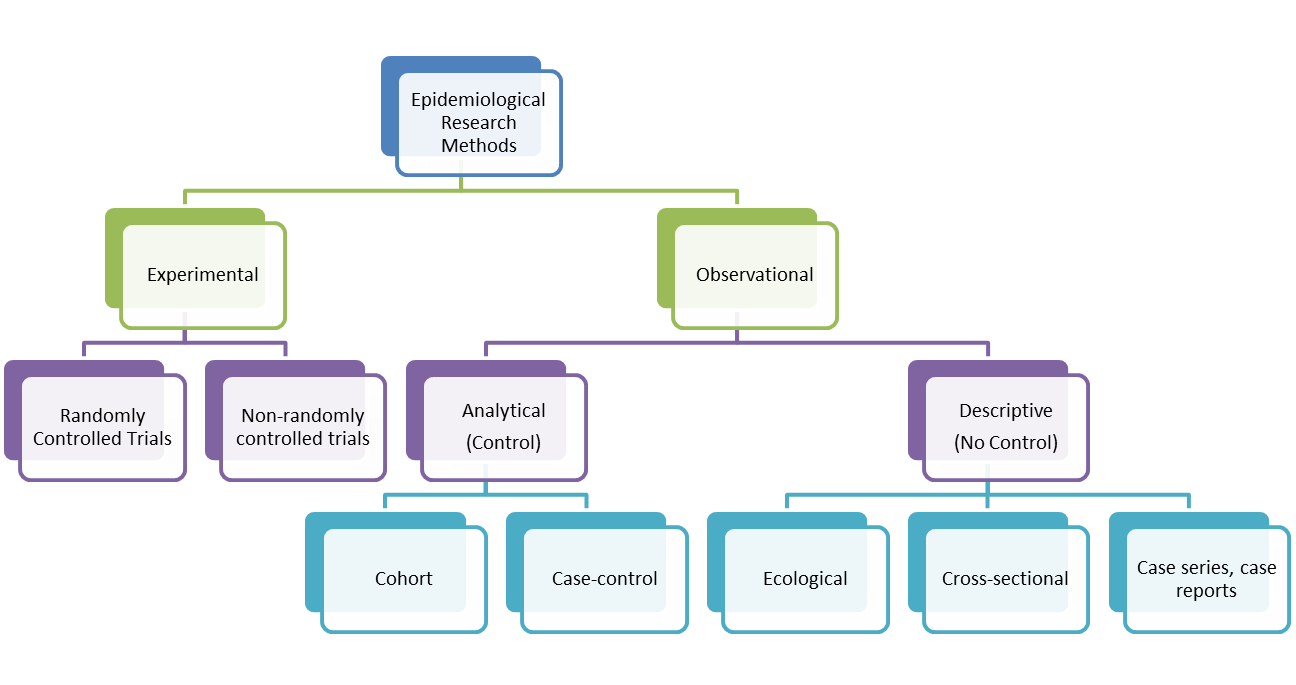
Descriptive studies are observational studies which describe the patterns of disease occurrence in relation to variables such as person, place and time. They are often the first step or initial enquiry into a new topic, event, disease or condition. Descriptive studies can be divided into two roles - those studies that emphasize features of a new condition and those which describe the health status of communities or populations. Case reports, case-series reports, before-and-after studies,cross-sectional studies and surveillance studies deal with invidiuals. Ecological Studies examine populations. Common misuses of descriptive studies involve a lack of a clear, specific and reproducible case definition and establishing a casual relationship which the data cannot support. Whilst descriptive studies can highlight associations between variables or between exposure and outcome variables, they cannot establish causality. Descriptive studies do not have a comparison (control) group which means that they do not allow for inferences to be drawn about associations, casual or otherwise. However, they can suggest hypotheses which can be tested in analytical observational studies.
Uses of Descriptive Studies
1. Health care planning
Descriptive studies provide knowledge about which populations or subgroups are most or least affected by disease. This enables public health administrators to target particular segments of the population for education or prevention programmes and can help allocate resources more efficiently.
2. Hypothesis generation
Descriptive studies identify descriptive characteristics which frequently constitutes an important first step in the search for determinants or risk factors that can be altered or eliminated to reduce or prevent disease.
3. Trend Analysis
Time-trend analysis is a longitudinal descriptive study that can provide a dynamic view of a population's health status. Data is collected over time, place and person to look for trends and changes.
Types of Descriptive Studies
Case reports
Case reports describe the experience of a single patient or a group of patients with a similar diagnosis. These types of studies typically depict an observant clinician identifying an unusual feature of a disease or a patient's history. They can represent the first clues in the identification of new diseases or adverse effects of an exposure. A case report can prompt further investigations with more rigorous study design. Case reports are quite common in medical journals. A systematic review found that they accounted for over one third of all articles published. They are useful to public health as they can provide an interface between clinical medicine and epidemiology.
Case Series
A case series is a report that describes clinical findings seen in a succession of patients who seem to display a similar condition or an outcome of interest. Another way of defining a case series is that case series are collections of individual case reports which may occur within a fairly short period of time and these are aggregated into one publication. No control group is involved. Something unexpected has been observed - e.g. more cases than usual of a rare disorder or new signs and symptoms of an emerging disease - hence the motivation to write it up and share it with the wider clinical community.
This study design has historical importance in epidemiology. It was often used as an early means to identify the begining or presence of an epidemic. Even now, the routine surveillance of accumulating case reports often suggest the emergence of a new disease or epidemic. A convenient feature of case-series is that they can provide a case group for a case-control study. An advantage of case series over case report is that a case series can help formulate a new and useful hypothesis rather than merely documenting an interesting medical oddity. However, its disadvantage is that it cannot be used to test for the presence of a valid statistical association.
Cross-sectional (Prevalence) Study
This is the observation of a defined population at a single point in time or time interval. Exposure and outcome are determined simultaneously. The cross-sectional study describes the presence and/or absence of various clinical features, so it provides a cross-sectional comparison. This means that costs are small and loss to follow up is not a problem. However, because exposure and outcome are measured at the same time point, the temporal sequence is often impossible to determine. Sometimes the cross-sectional study can be considered an analytic study, when it is used to test an epidemiologic hypothesis. This can only occur when the current values of the exposure variables are unaltered over time, thus representing the value present at the initiation of the disease. For example, factors at birth.
The cross-sectional survey is sometimes referred to as a prevalance study and it can survey or assess the health status of a population - e.g. Health Survey of England. A survey can be defined as a special inquiry which collects planned information from individuals (usually a sample) about their history, habits, knowledge, attitudes or behaviour. The principles involved include sampling, instrument design, non-response and accuracy. Reasons for non-response incorporate the effect of the topic, study design (postal, telephone or face-to-face interviews), age, sex, social class, urban/rural location and general attitudes to survey. See entry on Survey in Toolkit for more details.
It is worth noting that the term 'cross-sectional' study is also used in social research. Here, the cross-sectional study refers to a snapshot of a population at a particular point in time. This contrasts with longitudinal studies which follow a population over a period of time (i.e. cohort and panel), with cross-comparative, where one population is compared with another within the same country and cross-national, where one country population is compared with other countries.
Ecological Study (or Ecological Correlational Study)
Ecological correlational studies look for associations between exposures and outcomes in populations rather than in individuals. They use data that has already been collected. (This could be argued to be a form of what social scientists call secondary statistical analysis). The measure of association between exposure and outcome is the correlation coefficent r. This is a measure of how linear the relationship is between the exposure and outcome variables. (Note that correational is a specific form of association and requires two continuous variables).
Advantages of an ecological study
- An ecological study is quick and cheap to conduct.
- It can generate new hypotheses.
- It can identify new risk factors.
Disadvantages of an ecological study
- It is unable to control for confounding factors. This is often referred to as 'ecological fallacy', where two variables seem to be correlated but their relationship is in fact affected by cofounding factor(s).
- It cannot link exposure with disease in individuals as those with disease may not be expose.
- Its use of average exposure levels masks more complicated relationships with disease.
- Its units of study are populations not individuals. Therefore, the disease rates linked with population characteristics and the association observed at group level does not reflect association at individual level.
A useful way to remember the disadvantages is the acronym 'CLAP' - confounding, link, average and population.
Further Reading
Grimes, D.A. & Schultz, K.F. (2002) "Descriptive Studies: what they can and cannot do". The Lancet, 359, 145-49.